Transition metal catalysis
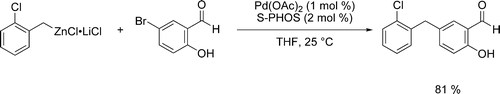
Pd-catalyzed cross-couplings that allow linking of unsaturated molecular fragments are of central importance in synthetic organic chemistry. The resulting products are highly relevant for applications in the pharmaceutical or agrochemical industry, as well as for the preparation of new materials.
We developed reaction conditions and a catalytic system allowing an efficient cross-coupling between various organozinc reagents and a broad range of aryl halides bearing relatively acidic NH or OH groups.
- Manolikakes, Georg; Schade, Matthias A.; Mu ñoz Hernandez, Carmen; Mayr, Herbert; Knochel, Paul. Negishi Cross-Couplings of Unsaturated Halides Bearing Relatively Acidic Hydrogen Atoms with Organozinc Reagents. Org. Lett., 2008, 10, 2765-2768.
- Manolikakes, Georg; Mu ñoz Hernandez, Carmen; Schade, Matthias A; Metzger, Albrecht; Knochel, Paul. Palladium- and Nickel-Catalyzed Cross-Couplings of Unsaturated Halides Bearing Relatively Acidic Protons with Organozinc Reagents. J. Org. Chem., 2008, 73, 8422-8436.
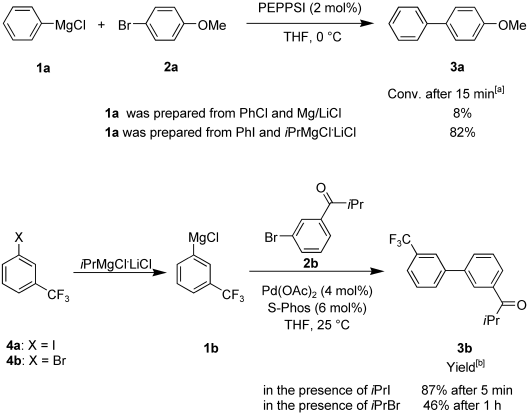
The Kumada cross-coupling allows direct Pd-catalyzed carbon-carbon bond formation between unsaturated halides and organomagnesium reagents (without further transmetalation steps) and is therefore a highly atom-economical cross-coupling reaction.
We found a new radical catalysis which makes it possible to perform a Kumada cross-coupling using aryl bromides at room temperature within a few minutes in the presence of an alkyl iodide.
- Manolikakes, Georg; Knochel, Paul. Radical Catalysis of Kumada Cross-Coupling Reactions Using Functionalized Grignard Reagents. Angew. Chem. Int. Ed., 2009, 48, 205-209.
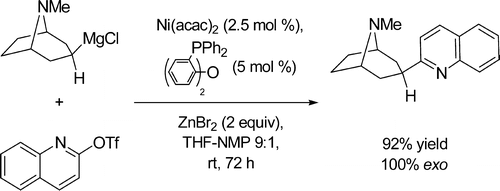
A direct room-temperature Ni-catalyzed cross-coupling of aminoalkylzinc halides, readily available from the corresponding aminoalkyl chlorides via Grignard reagents, with aryl and hetaryl electrophiles, allows a convenient one-step preparation of aminoalkyl (het)arenes, bearing a basic tertiary nitrogen in the side chain, including piperidine and tropane derivatives. Such aminoalkylarene scaffolds are widely present in various biologically active molecules.
- Melzig, Laurin; Gavryushin, Andrey; Knochel, Paul. Direct Aminoalkylation of Arenes and Hetarenes via Ni-Catalyzed Negishi Cross-Coupling Reactions. Org. Lett., 2007, 9, 5529-5532.
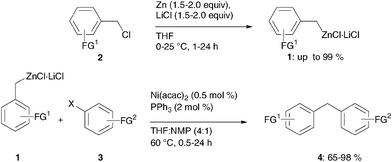
Benzylic zinc reagents prepared by direct insertion of zinc to benzylic chlorides in the presence of LiCl undergo smooth cross-coupling reactions with aromatic chlorides, bromides and tosylates using Ni(acac)2 and PPh3 as a catalyst system.
- Schade, Matthias A.; Metzger, Albrecht; Hug, Stefan; Knochel, Paul. Nickel-catalyzed cross-coupling reactions of benzylic zinc reagents with aromatic bromides, chlorides and tosylates. ChemComm, 2008, 3046-3048.




