Indium reagents
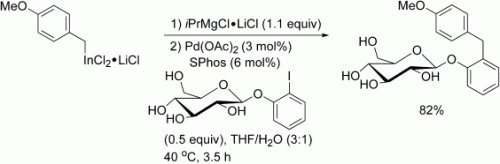
Sensitive functional groups such as COR, CHO, or CH2OH can be present in benzylic indium reagents prepared by the direct insertion of indium in the presence of LiCl. These reagents undergo palladium-catalyzed cross-coupling reactions in the presence of a protic cosolvent after activation with iPrMgClLiCl (see scheme). Remarkable chemoselectivities are achieved by using various electrophiles containing NH or OH groups.
- Chen, Yi-hung; Sun, Mai; Knochel, Paul. LiCl-Mediated Preparation of Functionalized Benzylic Indium(III) Halides and Highly Chemoselective Palladium-Catalyzed Cross-Coupling in a Protic Cosolvent. Angew. Chem. Int. Ed., 2009, 48, 2236-2239.
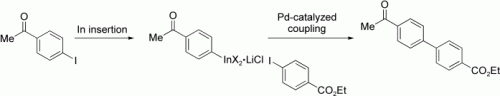
Sensitive functional groups, including ketone, aldehyde, and ester groups, may be present in aryl indium reagents prepared in good to excellent yields by the treatment of aryl and heteroaryl iodides with indium powder in the presence of LiCl (see example). These functionalized organoindium(III) reagents readily undergo Pd-catalyzed cross-coupling with functionalized aryl iodides, including those containing NH or OH groups.
- Chen, Yi-Hung; Knochel, Paul. Preparation of Aryl and Heteroaryl Indium(III) Reagents by the Direct Insertion of Indium in the Presence of LiCl. Angew. Chem. Int. Ed., 2008, 47, 7569-7580.




