Asymmetric Synthesis
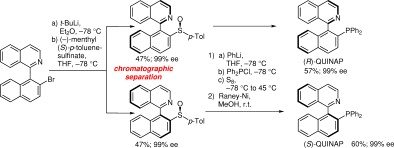
A novel entiaoselective synthesis of chiral QUINAP is described. Hereby, the seperation of the disastereomers was achived by the preparation and simple chromatographic seperation of chiral sulfoxide intermediates. Subsequent sulfoxide-lithium exchange, quenching with Ph2PCl and sulfur, then desulfurisation with Raney-Ni provided (R)- and (S)-QUINAP in 54-56% overall yield.
- Thaler, Tobias; Geittner, Paul; Knochel, Paul. A Novel Synthetic Approach towards Chiral QUINAP via Diastereomeric Sulfoxide Intermediates. Synlett, 2007, 17, 2655-2658.




